Resource Pages
Sep 28, 2018
EPA Meets Important TSCA Milestone by Finalizing Last of Four Chemical Safety Framework Rules
fees rule under the Toxic Substances Control Act (TSCA), ensuring that
resources are available to the Agency to complete chemical reviews and
actions in a timely, transparent manner while maintaining high
scientific standards.
"With today's action EPA has once again met another important
milestone under TSCA," said EPA Acting Administrator Andrew Wheeler.
"This rule will provide resources needed to support the valuable work
EPA does to review chemicals for safety, manage risk as required, and
make chemical information available as appropriate."
"The SBA Office of Advocacy was pleased to be able to work with EPA
and the SBA Office of Size Standards to develop the fees rule for the
administration of the Toxic Substance Control Act and to be able to
assist the agency in revising the small business definition to ensure
that the maximum number of small businesses can benefit from a reduced
fee amount," said Small Business Administration Office of Advocacy
Acting Chief Counsel Major L. Clark III.
These fees collected from chemical manufacturers will go toward
developing risk evaluations for existing chemicals; collecting and
reviewing toxicity and exposure data and other information; reviewing
Confidential Business Information (CBI); and making determinations in
a timely and transparent manner with respect to the safety of new
chemicals before they enter the marketplace. Under the final rule,
affected businesses will begin incurring fees on October 1, 2018.
Small businesses will be eligible to receive a substantial discount of
approximately 80% on their fees.
The fees rule is the last of four framework rules under the Frank R.
Lautenberg Chemical Safety for the 21st Act, incorporating input
received through public meetings and comment periods. During fiscal
years 2019-2021 the Agency will work to track costs and will use that
information to adjust future fees, if appropriate. As required by law,
EPA will evaluate and readjust, if necessary, the fees every three
years.
Additionally, EPA will host a series of webinars focusing on making
TSCA submissions and paying fees under the final rule. The webinars
will be held on October 10, October 24, and November 7.
Learn more about the webinars and register to attend.
https://www.epa.gov/tsca-fees/webinars-tsca-administration-fees-rule
Background on The Trump Administration's Lautenberg Act Accomplishments
The Trump Administration through its work at EPA has undertaken many
implementation activities which have enabled the agency to meet its
statutory responsibilities under the Frank R. Lautenberg Chemical
Safety for the 21st Century Act which amends the Toxic Substances
Control Act, the Nation's primary chemicals management law.
EPA has already met critical initial deadlines from the Lautenberg
Act, including issuing the framework rules on existing chemicals
prioritization, risk evaluation,
existing chemicals inventory by the first-year anniversary of the Act
on June 22, 2017.
On the second-year anniversary of the Lautenberg Act, EPA issued:
- Mercury Use Reporting Rule with deadlines and requirements to assist
in updating the inventory of mercury supply, use, and trade in the
United States.
- Alternative Testing Strategy to promote the development of
alternative test methods to reduce vertebrate animal testing. On March
7, 2018, EPA released the draft strategy for public comment.
- Guidance on Generic Names to allow EPA to share more information
with the public about the structure of chemicals while protecting CBI.
- Policy on Assigning Unique Identifiers to better publicly track
information on chemicals while protecting CBI.
Guidance on Expanding CBI access to states, tribes, and local
governments; health and environmental professionals; and first
responders.
For more information on TSCA implementation, visit:
https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/frank-r-lautenberg-chemical-safety-21st-century-act-5
Delaware to Distribute Potassium Iodide (KI) Tablets in a program initiated by the U.S. Nuclear Regulatory Commission.
October 4, 2018....
The State of Delaware received the tablets when it chose to
participate in a program initiated by the U.S. Nuclear Regulatory
Commission.
Potassium iodide does not protect against external radiation, but can
help protect the thyroid gland from ingested or inhaled radioactive
iodine that might be released in a radiation emergency. KI is one of
the measures outlined in Delaware's emergency plans developed for use
in a nuclear incident.
In the event of a radiation emergency, state and local officials will
inform the public through an Emergency Alert System (EAS) message via
local radio stations, which will include instructions on how affected
residents should react, directions to evacuation routes and emergency
reception centers, and if or when to take the KI tablets.
Those who are eligible to receive potassium iodide should bring a
photo ID such as a driver's license, proof of residency such as a
utility bill, or proof of employment within the EPZ, when they go to
the distribution center at Middletown Fire Hall. Individuals in
possession of potassium iodide that has passed the expiration date can
bring those tablets to the distribution center to exchange for new
tablets.
Delaware residents living outside of the 10-mile EPZ who would like to
obtain potassium iodide tablets should contact their pharmacist. KI
is available over-the-counter at some local pharmacies.
Read full from Source:
https://news.delaware.gov/2018/09/27/delaware-distribute-potassium-iodide-ki-tablets-3/
Sep 27, 2018
EPA Publishes SNURS for 28 Chemical Substances
(ACA - Paint.org) The U.S. Environmental Protection Agency (EPA) on Sept. 17 published in the Federal Register its intent to promulgate significant new use rules (SNURs) under the Toxic Substances Control Act (TSCA) for 28 chemical substances that were the subject of premanufacture notices (PMNs).
The SNURs are effective on Nov. 16, 2018.
Under the amended TSCA, persons who intend to manufacture (defined by statute to include import) or process any of these 28 chemical substances for an activity that is designated as a significant new use by this rule, are required to notify EPA at least 90 days before commencing that activity. The required notification initiates EPA's evaluation of the intended use within the applicable review period.
Persons may not commence manufacture or processing for the significant new use until EPA has conducted a review of the notice, made an appropriate determination on the notice, and has taken such actions as are required with that determination.
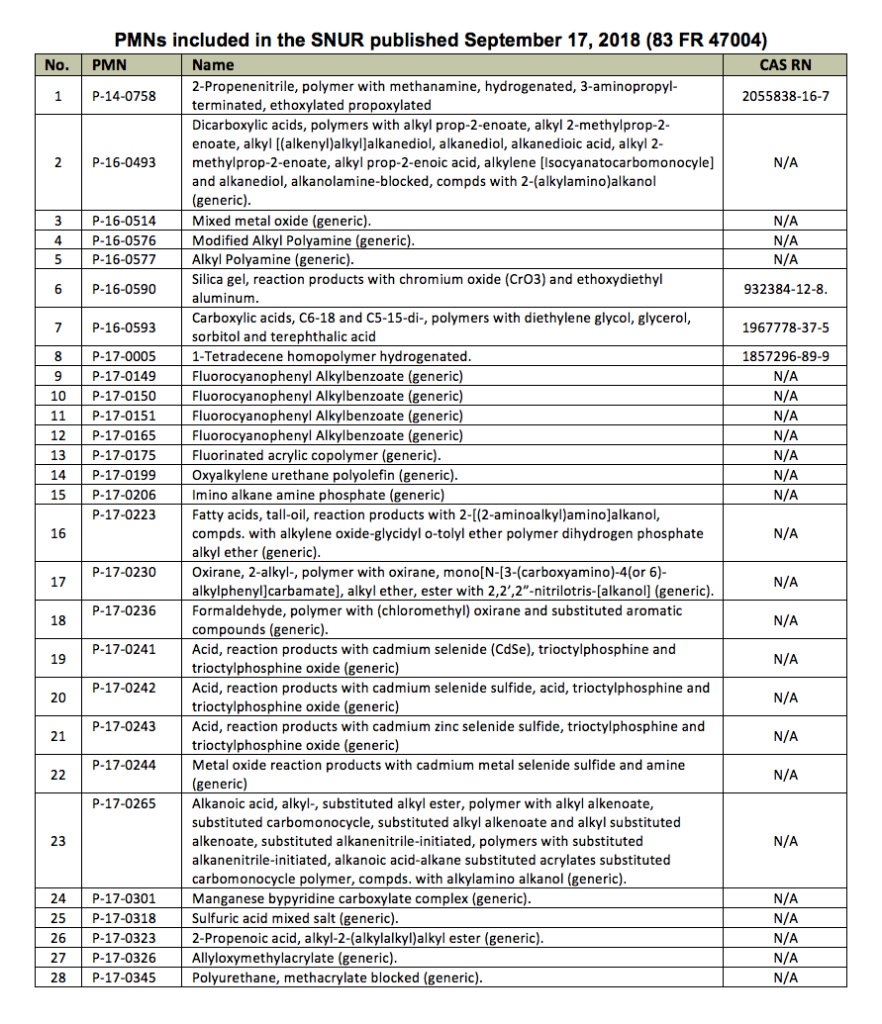
The SNURs may contain restrictions on use and discharge of chemical substances, Personal Protective Equipment, and record-keeping, among other requirements.
EPA is accepting comments objecting to one or more of these SNURs through Oct. 17, 2018. If EPA receives written adverse comments, on one or more of these SNURs by the deadline, the agency will withdraw the relevant sections of this direct final rule before its effective date. In effect, EPA will withdraw any SNURs subject to adverse comment from the direct final rule. EPA would then enter a separate rulemaking procedure with notice and comment about these SNURs.
Notably, this process for identifying SNURS via PMNs, as EPA is currently implementing is the basis for the NRDC (Natural Resources Defense Council) withdrawing its lawsuit against EPA, late this summer. NRDC claimed procedures outlined in EPA's revised guidance for review of New Chemicals violates regulations under TSCA, to the extent EPA would issue SNURs as an outcome of the PMN review process. EPA states in guidance that it would address reasonably foreseen uses not identified by a PMN applicant by issuing a SNUR while conducting PMN review.
NRDC was concerned that conflating the SNUR and PMN process into one procedure could lead to insufficient data to evaluate reasonably foreseen uses. NRDC argued that TSCA requires EPA issue a consent letter to a PMN applicant evaluating uses in the PMN application. EPA can then evaluate any reasonably foreseen uses not identified by the applicant with a SNUR, through a separate rulemaking process as necessary.
The bill, co-written by Congresswoman Susan Brooks, would streamline the process how hospitals gets money to treat those who have contracted diseases such as Ebola.
Sep 19, 2018
EPA Extends Comment Period for Proposal to Reconsider RMP Rule Amendments
(ACA) On July 24, the U.S. Environmental Protection Agency (EPA) published in the Federal Register a notice of data availability and extension of the comment period for its May 30 proposed rule to reconsider the final Risk Management Program (RMP) amendments. The extension follows an inquiry from nonprofit EarthJustice, which questions 2017 RMP data that EPA collected from industrial facilities and used to roll back the RMP amendments.
EPA officially finalized the RMP amendments on Jan. 13, 2017; however, the effective date of the final RMP amendments was delayed several times, with the last one delaying the amendments until Feb.19, 2019. Since the final RMP amendments have not become effective yet, EPA is now proposing to rollback several provisions of that 2017 RMP rule. Among others, EPA is proposing to rescind amendments relating to third-party compliance audits, safer technology and alternatives analyses, incident investigations, and information availability to the public. In addition, EPA is also proposing to retain, modify, or incorporate some amendments relating to local emergency response coordination, emergency response exercises, and CBI protections. More information can also be found on EPA's website.
The agency is now accepting comments on its proposal through Aug. 23, 2018. ACA is seeking member input on any concerns with EPA's latest proposal.
In general, EPA's RMP program applies to all stationary sources with processes that contain more than a threshold of a regulated substance. The program's elements are intended to prevent accidental releases and reduce the severity of releases that occur. All sources must prepare and submit an RMP to EPA at least every five years. In addition, RMP Program 3 facilities involve processes subject to OSHA's Process Safety Management (PSM) standard or are in one of the specified NAICS codes, such as chemical manufacturing. Together, PSM and RMP form the regulatory framework for prevention of catastrophic chemical accidents at fixed facilities. Several ACA companies have facilities subject to RMP requirements, particularly Program 3 facilities, which have the most stringent requirements. ACA's main concern with the 2017 RMP amendments was that the changes would not actually enhance chemical facility safety, but would instead create significant administrative burdens and higher compliance costs without commensurate benefits in safety.
In May 2017, ACA submitted comments to EPA in support of the agency's proposed rule to further delay the effective date of the RMP regulation. ACA underscored that during this proposed delay, the existing RMP regulations will remain in place, and noted that the already robust RMP requirements have resulted in a steady decline in reportable accidental releases over the past 20 years. From 2004 to 2013, EPA data shows that there were roughly 12,500 facilities subject to RMP. During that 10-year span, 92 percent of these facilities had no RMP reportable accidents. This decline in reportable accidental chemical releases is expected to continue under the existing RMP regulations.
The 2017 RMP rule amendments encountered extreme resistance since EPA first issued them in mid-January 2017. EPA stated that the amendments made to the final rule were aimed at modernizing RMP by (1) making changes to the accident prevention program requirements, (2) enhancing the emergency response and preparedness requirements, and (3) modifying the information availability requirements. However, numerous industry members and trade associations pushed back against implementation of these amendments.
OSHA Proposes Rule to Better Protect Personally Identifiable Information
(ACA) On July 30, the U.S. Occupational Safety and Health Administration (OSHA) published in the Federal Register, a Notice of Proposed Rulemaking to better protect personally identifiable information or data that could be re-identified with a particular individual by removing provisions of the "Improve Tracking of Workplace Injuries and Illnesses" rule. OSHA believes that this proposal maintains safety and health protections for workers while also reducing the burden to employers of complying with the current rule.
The proposed rule eliminates the requirement to electronically submit information from OSHA Form 300 (Log of Work-Related Injuries and Illnesses), and OSHA Form 301 (Injury and Illness Incident Report) for establishments with 250 or more employees that are currently required to maintain injury and illness records. These establishments would be required to electronically submit information only from OSHA Form 300A (Summary of Work-Related Injuries and Illnesses). In addition, OSHA is proposing to require covered employers to submit their Employer Identification Number (EIN) electronically along with their injury and illness data submission.
In the May 2016 "Improve Tracking of Workplace Injuries and Illnesses" final rule (81 FR 29624), the recordkeeping regulation was revised to require establishments with 250 or more employees to electronically submit information from the OSHA Forms 300, 300A, and 301 to OSHA annually. Establishments in certain industries with 20-249 employees are required only to electronically submit information from only the OSHA Form 300A — the summary form. This proposed rule would amend OSHA's recordkeeping regulation by rescinding the requirement for establishments with 250 or more employees to electronically submit information from the OSHA Forms 300 and 301 — the individual forms.
OSHA is seeking comment on this proposal, particularly on its impact on worker privacy, including the risks posed by exposing workers' sensitive information to possible FOIA disclosure. OSHA will be accepting comments on the proposed rulemaking through Sept. 28, 2018.
OSHA's regulation at 29 CFR part 1904 requires employers to collect a variety of information on occupational injuries and illnesses. Much of this information may be sensitive for workers, including descriptions of their injuries and the body parts affected. Under OSHA's regulation, employers with more than 10 employees in most industries must keep those records at their establishments. Employers covered by these rules must record each recordable employee injury and illness on an OSHA Form 300, the "Log of Work-Related Injuries and Illnesses," or equivalent. Covered employers must also prepare a supplementary OSHA Form 301, the "Injury and Illness Incident Report" or equivalent, to provide additional details about each case recorded on the OSHA Form 300. OSHA requires employers to provide these records to others under certain circumstances but imposes limits on the disclosure of personally identifying information. Finally, at the end of each year, these employers are required to prepare a summary report of all injuries and illnesses on the OSHA Form 300A, the "Summary of Work-Related Injuries and Illnesses," and post the form in a visible location in the workplace.
Form 301 requires the collection of much sensitive information about each individual worker's job-linked illness or injury, information an employer must collect with or without the worker's consent. While some of the information is likelier to be regarded as particularly sensitive.
Form 300 requires employers to log much of this individual information — notably, descriptions of injuries and the body parts affected — for each individual worker and incident. Form 300A, by contrast, merely summarizes incident data without any traceable connection to individual workers.
Under the current recordkeeping rule, the deadline for electronic submission of Calendar Year (CY) 2017 information from OSHA Forms 300 and 301 was July 1, 2018. In subsequent years, the deadline is
March 2. OSHA is not currently accepting the Form 300 or 301 data and will not enforce the deadlines for these two forms without further notice while this rulemaking is underway. The electronic portal collecting Form 300A data is accepting CY 2017 data, although submissions after July 1, 2018, will be marked late.
California DTSC Proposes Priority Product for Paint and Varnish Strippers and Graffiti Removers Containing NMP
(ACA) On Aug.28, the California Department of Toxic Substances Control (DTSC) proposed listing paint and varnish strippers and graffiti removers containing 1-methyl-2-pyrrolidone (NMP) as Priority Products under its Safer Consumer Products Regulations. DTSC issued a Draft Product-Chemical Profile, which describes the information upon which it relied in making the determination that this product-chemical combination meets the identification and prioritization factors outlined for the Regulations: (1) there is potential for human and other organism exposure to NMP in paint and varnish strippers and graffiti removers; and (2) the exposure has the potential to contribute to or cause significant or widespread adverse impacts. Comments on the draft profile are due to DTSC by Oct. 1 via a portal at CalSAFER.
If listed as a priority product, companies must comply with requirements for reporting and identifying alternatives pursuant to the SCP regulations.
The California Safer Consumer Products Regulations were finalized in October 2013. The first category of products listed in July 2017 is children's foam-padded sleeping products containing certain flame retardants. The second category, spray polyurethane foam with unreacted MDI, was just listed in July 2018. Since no one manufactures the sleeping products and the spray polyurethane foam listing is brand new, no business has implement the voluminous analysis required by the regulations. The other two product categories, carpets and rugs with PFAS and laundry detergents with NPE, are still in the proposal phase.
Paint and varnish stripper products containing 1-methyl-2-pyrrolidone (NMP) are proposed as the fifth category of products to be regulated as a "Priority Product" under the California Safer Consumer Product Regulations.
DTSC will be hosting a public workshop to summarize the draft profile on Sept. 18, 2018 from 9:00 AM to 12:00 PM (PT) in Room 550 in the Cal EPA building. The hearing will be broadcast via webinar, as well, but participants must pre-register by clicking here.
Notably, exact data about amount of paint strippers with NMP sold in California is not available. A survey shows methylene chloride paint strippers are more commonly sold, but a number of paint strippers also use NMP as a methylene chloride substitute. NMP is a HPV (High Production Volume) chemical according to the U.S. Environmental Protection Agency (EPA), with 194.7 million pounds manufactured or imported in the United States in 2012.
Read full at: https://www.paint.org/dtsc-nmp/
Senate Introduces Bill to Reauthorize CFATS program for Five Years
(ACA) On Sept. 4, a bill to reauthorize for five years the Chemical Facility Anti-Terrorism Standards (CFATS) program, administered by the U.S. Department of Homeland Security (DHS), was introduced in the U.S. Senate. S. 3405, The Protecting and Securing Chemical Facilities from Terrorist Attacks Act, would extend CFATS beyond the current January 2019 sunset date. A companion bill in the U.S. House of Representatives has yet to be introduced.
CFATS is an important program aimed at preventing chemicals from being stolen, diverted, sabotaged, or deliberately released by terrorist or other bad actors. DHS CFATS regulations were issued as a final rule in November 2007; however, DHS implements the CFATS program under a variety of short-term authorizations by Congress.
Under CFATS, chemical facilities possessing more than a threshold amount of specific explosive, toxic, or other "chemicals of interest" determined by DHS, are required to complete a "top-screen," notifying DHS that they possess such chemicals on site. Once a facility submits its top-screen, DHS can direct the facility to submit a Security Vulnerability Assessment (SVA). The SVA provides the basis for DHS to assign the facility to one of four tiers: Tiers 1 and 2 being the highest risk, and Tiers 3 and 4 being the lowest. Tier assignment triggers a requirement to submit a Site Security Plan (SSP) or an Alternative Security Plan (ASP) to DHS for authorization and approval.
CFATS currently covers approximately 3,400 chemical facilities, which have been assessed to present a risk of terrorist attack or exploitation.
In addition to reauthorizing CFATS for five years, the proposed legislation also reforms the current CFATS program in the following ways:
- Grants a request by the explosives industry to be exempted from the requirements in favor of existing regulations by the federal Bureau of Alcohol, Tobacco, Firearms and Explosives;
- Prevents DHS from inspecting a facility more often than once every two years, or every three years if it's in a new voluntary recognition program;
- Allows Tier 3 and Tier 4 facilities to opt out from a requirement that they submit employee information to be screened for terrorist ties; and
- Requires DHS to conduct a formal rulemaking if it adds new chemicals to the program and would give facility operators more information explaining how they were evaluated for security risk.
These changes comport with some of the effective solutions ACA suggested to implement and improve chemical security. ACA submitted recommendations to Congress for CFATS enhancements compiled from ACA's member companies, who own and operate paint, coatings, resin, or chemical manufacturing facilities. Some of these facilities are subject to CFATS, with a clear majority being classified as Tier 4 facilities, while just a few are Tier 3.
EPA and U.S. Army Corps of Engineers Seek to Repeal 2015 WOTUS Definition
Sep 18, 2018
Hurricane Florence—Clinical Guidance for Carbon Monoxide (CO) Poisoning
Summary
The Centers for Disease Control and Prevention (CDC) is reminding clinicians seeing patients from the areas affected by Hurricane Florence to maintain a high index of suspicion for CO poisoning. Other people who may be exposed to the same CO source may need to be identified and assessed.
The signs and symptoms of CO exposure are variable and nonspecific. A tension-type headache is the most common symptom of mild CO poisoning. Other symptoms may include dizziness, flu-like symptoms without a fever, drowsiness, chest pain, and altered mental status.
Clinical manifestations of severe CO poisoning include tachycardia, tachypnea, hypotension, metabolic acidosis, dysrhythmias, myocardial ischemia or infarction, noncardiogenic pulmonary edema, neurologic findings including irritability, impaired memory, cognitive and sensory disturbances, ataxia, altered or loss of consciousness, seizures, coma, and death, although any organ system might be involved.
Although CO poisoning can be fatal to anyone, children, pregnant women, the unborn, persons with sickle cell disease, older adults, and persons with chronic illness (e.g., heart or lung disease) are particularly vulnerable.
Background
High winds and heavy rain from Hurricane Florence began affecting the southeastern U.S. around September 12, 2018. Impact on the southeast coast and inland led to thousands of people without power. Those without power may turn to alternate power sources such as gasoline generators and may use propane or charcoal grills for cooking. If used or placed improperly, these sources can lead to CO build up inside buildings, garages, or campers and poison the people and animals inside.
With a focused history of patient activities and health symptoms, exposure to a CO source may become apparent. Appropriate and prompt diagnostic testing and treatment are crucial to reduce morbidity and prevent mortality from CO poisoning. Identifying and mitigating the CO source is critical in preventing other poisoning cases.
Recommendations for Clinicians
- Consider CO poisoning in patients affected by Hurricane Florence, particularly those in areas currently without power. Assess symptoms and recent patient activities that point to likely CO exposure. Evaluation should also include examination for other conditions, including smoke inhalation, trauma, medical illness, or intoxication.
- Administer 100% oxygen until the patient is symptom-free or until a diagnosis of CO poisoning has been ruled out.
- Perform COHgb testing when CO poisoning is suspected. Venous or arterial blood may be used for testing. A fingertip pulse multiple wavelength spectrophotometer, or CO-oximeter, can be used to measure heart rate, oxygen saturation, and COHgb levels in the field, but any suspicion of CO poisoning should be confirmed with a COHgb level by multiple wavelength spectrophotometer (CO-oximeter). A conventional two-wavelength pulse oximeter is not accurate when COHgb is present. For more information, see https://www.cdc.gov/disasters/
co_guidance.html . - An elevated carboxyhemoglobin (COHgb) level of 2% or higher for non-smokers and 9% or higher COHgb level for smokers strongly supports a diagnosis of CO poisoning. The COHgb level must be interpreted in light of the patient's exposure history and length of time away from CO exposure, as levels gradually fall once the patient is removed from the exposure. In addition, carbon monoxide can be produced endogenously as a by-product of heme metabolism. Patients with sickle cell disease can have an elevated COHgb level as a result of hemolytic anemia or hemolysis. For additional information about interpretation of COHgb levels, visit https://www.cdc.gov/disasters/
co_guidance.html or call Poison Control at (800) 222-1222. - Hyperbaric oxygen therapy (HBO) should be considered in consultation with a toxicologist, hyperbaric oxygen facility, or Poison Control Center (800) 222-1222. For additional management considerations, consult a toxicologist, Poison Control at (800) 222-1222, or a hyperbaric oxygen facility.
- Be aware that CO exposure may be ongoing for others spending time in or near the same environment as the patient. These individuals should be evaluated and tested as described in this advisory.
- Clinicians treating people for CO poisoning should notify emergency medical services (EMS), the fire department, or law enforcement to investigate and mitigate the source and advise people when it is safe to return.
- Advise patients about safe practices related to generators, grills, camp stoves, or other gasoline, propane, natural gas, or charcoal-burning devices. Stress that that these devices should never be used inside an enclosed space, home, basement, garage, or camper — or even outside near an open window or window air conditioner. Please see https://www.cdc.gov/co/pdfs/
generators.pdf .
For More Information
Clinical Guidance for Carbon Monoxide (CO) Poisoning After a Disaster
https://www.cdc.gov/disasters/