We humans like to think of ourselves as the pinnacle of evolution on the planet, but that's just a conceit. It takes humans roughly twenty years to reproduce, whereas some bacteria can make copies of themselves every 20 minutes. Countless generations of bacteria have honed and perfected their genomes into extremely evolved biological machines.
Most bacteria are harmless, and some are quite useful, even tasty – witness the lactofermented pickles and sauerkraut I made this summer. But some bacteria are pathogenic nightmares that have swarmed over the planet and caused untold misery and billions of deaths. For most of human history it has been so – the bugs were winning. Then a bright period dawned in the early 20th century – the Era of Antibiotics. At last we were delivered from the threat of pestilence, never more to suffer from plague and disease like our unfortunate ancestors. Infections were miraculously cured with a simple injection or pill, childhood diseases were no longer reaping their tragic harvest, and soldiers on the battlefield were surviving wounds that would have festered and led to a slow, painful death.
Now it seems like this bright spot of relief from bacterial disease might be drawing to an end. Resistant strains of bacteria are in the news these days, and the rise of superbugs seems inevitable. But is it? Have we run out of tools to fight back? Not quite yet as it turns out. But there's a lot of work to do to make sure we win this battle.
Artificial Selection in Action
![Alexander Fleming [via Wikipedia]](https://hackadaycom.files.wordpress.com/2015/09/alexander_fleming.jpg?w=400&h=301)
In the 75 years since then, huge advancements in antibiotic therapy beyond penicillin have been made, and literally millions of lives have been saved by the tetracyclines, sulfonamides, cephalosporins and quinolones that have followed it. Each antibiotic class has its own method of action, and each medicine has a particular niche that it exploits – penicillins disrupt bacterial cell wall synthesis, for example, while tetracyclines inhibit protein synthesis in bacterial ribosomes. But each little trick that we exploit to kill off bacteria has an unintended consequence – it leaves survivors behind. Random mutations and genetic reassortment lead to the chance that a handful of cells in the infection will be able to resist the antibiotic being used. When the susceptible cells are killed off, the resistant cells are not only left behind, they're left with an open playing field and tons of resources to exploit. The survivors reproduce, pass their resistance on to the next generation, and pretty soon you've artificially selected a population of superbugs.
Obviously it's all a little more complicated than that. Our immune systems are hard at work during an infection, and once the susceptible cells are culled it generally does a pretty good job of mopping up the small number of resistant bacteria. And resistance does not necessarily mean "antibiotic-proof" – it might just mean a cell is able to put up more of a fight to the antibiotic of choice. This is the reason for long courses of antibiotic therapy – knock the infection back with the first dose or two, then keep the levels of the medicine up to keep up the pressure on the tougher bugs so the immune system can mop up. Quit the medicine early, and you're just giving the resistant bugs a chance to explode in population and cause a real problem.
Misuse of antibiotics has also contributed to the current state of affairs. Patients generally want to walk out of their doctor's office with something to show for the effort and expense, and being told to go home and wait out a viral infection often doesn't sit well. Going home with a useless course of antibiotics used to be a common practice and has no doubt contributed to the selection of the resistant strains of bacteria we're seeing today.
Ancient Wisdom
But isn't there a disconnect here? If we are so susceptible to bugs, and we only figured out how to fight them off less than a century ago, how did we even get this far as a species? While it's true that modern antibiotics are only a recent development, traditional treatment of infection goes back a lot further. Traces of tetracycline, which strongly binds to the mineral matrix of bones and teeth, can be found in human skeletal remains from 1700 years ago. It's not clear whether they were purposely dosing themselves or just picking up tetracycline from naturally occurring soil bacteria, but they were exposed and probably benefited from it.

What's Next?
As fruitful as it may prove to tap into ancient wisdom, the fact remains that the microbes will always be one step ahead of us. Whether we throw a new synthetic molecule or an ancient potion at an infection, random mutations and generation times measured in minutes will combine to quickly evolve resistance to the next therapy. But what if there was a way to strip antibiotic resistance from bacteria? Then no matter what new tricks they learn, we'd be able to sensitize them to antibiotics and maybe gain a little ground in the fight against infection.
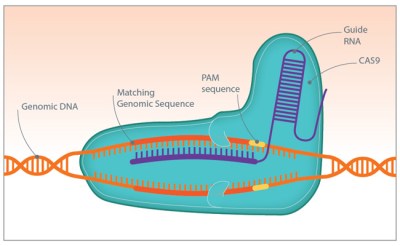
CRISPR is a really powerful tool for gene editing, and one that has applications for overcoming antibiotic resistance. In an ironic twist, researchers are packing CRISPR/Cas systems into phages and using them to attack bacteria. The CRISPR system is programmed to search for and destroy the sequences that code for antibiotic resistance, like the beta-lactamase protein that confers penicillin resistance. The bacteria are then vulnerable to antibiotics they had previously been able to stand up to.
There are limits to what CRISPR can accomplish in the fight against infection. One obvious problem is finding a phage that attacks each bug you want to kill, and being able to genetically modify it with a CRISPR payload. And the bugs still have the evolutionary upper hand, since they can rapidly try out random mutations that may overcome the CRISPR attack. But it's at least another tool for us to use, and one that allows us to rapidly respond to new threats. Moreover, it's a very approachable technology in terms of equipment and instrumentation. Indeed, CRISPR has become the hot new technique for biohackers who seek to create everything from better yeasts for craft brewed beers to vegan cheese. Perhaps some of these biohackers will turn their attention to the problem of antibiotic resistance too.
Even though the evolutionary deck is clearly stacked against us, I don't think we're done for quite yet. While the bugs have the numbers, we have our own evolutionary gift – a great big forebrain. If we put enough of them on the problem, some breakthrough will happen. So while the Era of Antibiotics isn't necessarily at an end, it probably is at a really interesting inflection point.
[MRSA SEM Image via Wikipedia]
Please continue reading from: Hack a DayFiled under: Featured, Medical hacks